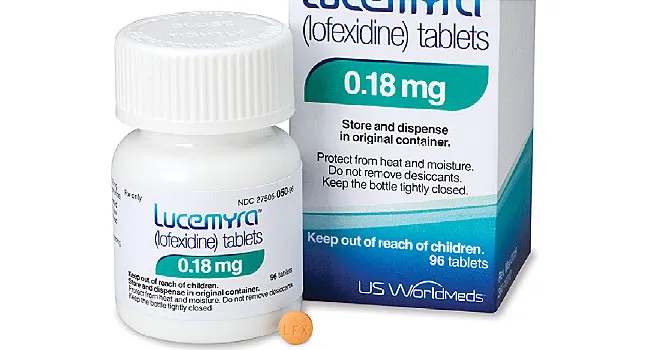
The FDA found the drug to be safe and effective in easing symptoms such as diarrhea, nausea, vomiting, anxiety, and an overall feeling of sickness that often keep patients from withdrawing from opioids.
From: https://www.webmd.com/mental-health/addiction/news/20180517/fda-approves-first-non-opioid-for-withdrawal?src=RSS_PUBLIC
No comments:
Post a Comment